Abstract
Background
Ravulizumab, a humanized anti-complement C5 monoclonal antibody designed by targeted modification of eculizumab to achieve an extended half-life, is approved to treat aHUS in the USA (2019), EU and Japan (2020). Data at 26 weeks (wk) and 1 year (yr) from the phase 3 studies of ravulizumab in adults and children with aHUS have been published. Here we report 2-yr data from these trials.
Methods
Efficacy and safety data from ravulizumab clinical trials in adults naïve to complement inhibitor treatment (NCT02949128), and in children either naïve to (naïve) or switched from (switch) eculizumab (NCT03131219), were assessed at 2 yr and presented alongside data from the initial 26-wk evaluation periods; patients were dosed every 8 wk (adult), or every 4 or 8 wk (children), according to body weight. Descriptive statistical analyses were conducted on these data. No statistical comparisons between data at 26 wk and 2 yr, or between trials, were conducted.
Results
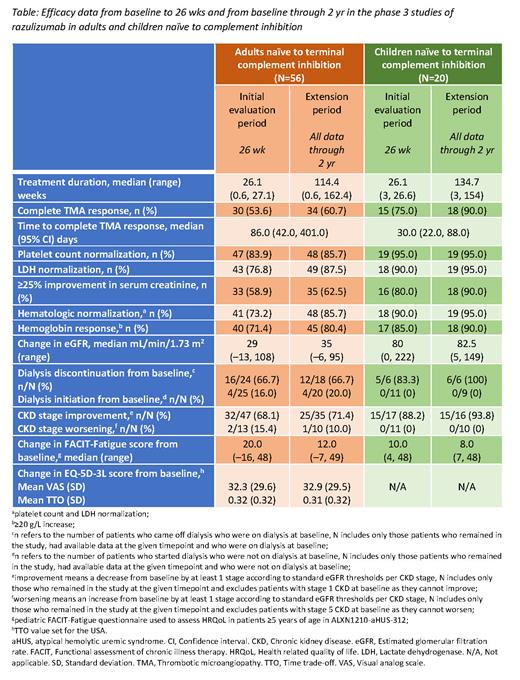
Efficacy data in adults and treatment-naïve children are presented in the Table. Complete thrombotic microangiopathy (TMA) response (platelet count normalization, lactate dehydrogenase normalization, and ≥25% improvement in serum creatinine from baseline, met concurrently at 2 separate assessments, at least 4 wk apart) was achieved in more patients at 2 yr vs 26 wk in both studies (adult: 34 [61%) vs 30 [54%]; naïve: 18 [90%] vs 15 [75%]). All complete TMA response components were either numerically improved or maintained at 2 yr vs 26 wk. Kidney function continued to improve, with the median change in estimated glomerular filtration rate from baseline numerically increasing at 2 yr vs 26 wk in both adults (35 vs 29 mL/min/1.73m 2) and naïve children (82.5 vs 80 mL/min/1.73m 2). Most patients receiving dialysis at baseline were able to discontinue dialysis at 26 wk; this was sustained in adults (67% vs 67%) while all naïve children receiving dialysis at baseline had discontinued by 2 yr (83% vs 100%). No patients who discontinued dialysis by 26 wk subsequently restarted. Chronic kidney disease (CKD) stage improved in most patients through 26 wk in both studies (adult, 68%; naïve, 88%); improvements were sustained at 2 yr (adult, 71%; naïve, 94%). No naïve children experienced a worsening of CKD stage at 2 yr. Improvements from baseline in quality of life were seen at both 26 wk and 2 yr, as measured by Functional Assessment of Chronic Illness Therapy-Fatigue (adults: 20 vs 12; naïve: 10 vs 8), EQ-5D-3L visual analog scale (adults: 32 vs 33) and EQ-5D-3L time trade-off (adults: 0.32 vs 0.31).
Most adverse events (AEs) and serious AEs (SAEs) occurring in these studies were reported during the initial 26-wk evaluation period, with a general reduction in the number of patients with any new S/AE events being reported at 2 yr. The most common AEs reported through 2 yr, which had not met the 15% reporting threshold through 26 wk, were (n, %): adults - constipation (9, 16%), fatigue (9, 16%) and nasopharyngitis (9, 16%); naïve children - abdominal pain (6, 25%), contusion (5, 21%), cough (5, 21%), nausea (4, 17%), myalgia (4, 17%), rash (4, 17%) and rhinorrhea (4, 17%); switch children - upper respiratory tract infections (URTI; 4, 40%), oropharyngeal pain (3, 30%), pharyngitis (3, 30%), cough (2, 20%), gastroenteritis (2, 20%), nasopharyngitis (2, 20%), otitis media (2, 20%), viral URTI (2, 20%) and dehydration (2, 20%). No patients discontinued either study due to treatment-emergent AEs after 26 wk. No meningococcal infections were recorded in either study at any timepoints. One adult patient, who had previously met complete TMA response criteria while receiving therapy, discontinued treatment by choice and subsequently experienced recurrent disease, as evidenced by an increase in serum creatinine (SCr). This patient then restarted therapy, leading to improvements in SCr levels.
Conclusions
In both treatment-naïve adults and children, ravulizumab was associated with numerically sustained or increased improvements in hematologic outcomes and kidney function at 2 yr vs 26 wk. Fewer S/AEs were reported during the extension period of these studies vs the initial 26-wk evaluation periods. Importantly, no meningococcal infections were reported in either study at any timepoint. The data suggest that long-term treatment with ravulizumab is well tolerated and may be associated with continuing improvements in TMA parameters and renal function in adults and children with aHUS.
Dixon: Apellis Pharmaceuticals: Consultancy; Horizon Pharmaceuticals: Consultancy; Alexion Pharmaceuticals: Consultancy. Kavanagh: Gyroscope Therapeutics: Current equity holder in publicly-traded company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Apellis: Honoraria, Speakers Bureau; Idorsia: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Alexion Pharmaceuticals: Honoraria, Speakers Bureau. Kang: Alexion Pharmaceuticals: Honoraria, Research Funding; Handok: Honoraria; Kyowa Kirin: Honoraria, Research Funding; Amgen: Research Funding; Bayer: Research Funding. Wang: Alexion, AstraZeneca Rare Disease Inc.: Current Employment. Garlo: Alexion: Current Employment; AstraZeneca: Current Employment. Greenbaum: Alexion Pharmaceuticals: Honoraria, Research Funding. Ogawa: Alexion Pharmaceuticals: Current Employment. Cataland: Ablynx/Sanofi: Consultancy, Research Funding; Sanofi Genzyme: Consultancy; Alexion: Consultancy, Research Funding; Takeda: Consultancy. Miyakawa: Sanofi: Consultancy; Zenyaku Kogyo: Consultancy; Sanofi: Research Funding; argenx: Consultancy, Research Funding.